The Connection Between Thyroid Disorders and Fertility
Mechanisms Linking Thyroid Dysfunction to Reproductive Outcome
Fertility depends on precise coordination between multiple hormonal systems. When that coordination is disrupted, conception may become difficult—even when reproductive organs appear structurally normal and menstrual cycles seem “regular.”
Thyroid dysfunction is one of the most common, yet frequently underrecognized, contributors to fertility challenges. Thyroid hormones influence ovulation, cycle regulation, implantation, and early pregnancy signaling. When thyroid function is impaired, fertility can be affected long before overt thyroid disease is diagnosed (1).
Importantly, this does not mean thyroid disorders make pregnancy impossible. It means thyroid signaling must be stable, responsive, and properly regulated for the reproductive system to function optimally.
Why Thyroid Function Matters for Fertility
Fertility depends on precise coordination between the brain, endocrine system, and reproductive organs. Thyroid hormones play a central regulatory role in this coordination by influencing metabolic rate, energy availability, and hormone signaling across reproductive tissues (1).
Rather than acting directly as “fertility hormones,” thyroid hormones function as permissive regulators. They help determine whether reproductive processes—such as ovulation, endometrial preparation, and early pregnancy signaling—can proceed efficiently under current physiological conditions.
When thyroid signaling is impaired, the body may shift away from reproduction as a priority, even in the absence of overt thyroid disease.
→ Women’s Hormone & Reproductive Health
Thyroid Hormones as Metabolic Gatekeepers
Reproduction is metabolically demanding. Ovulation, implantation, and early pregnancy all require sufficient cellular energy, oxygen utilization, and nutrient availability. Thyroid hormones regulate these processes by controlling how efficiently cells generate and use energy (1).
When thyroid hormone activity is reduced, the body may interpret this state as unfavorable for reproduction.
This can result in:
Subtle suppression of ovulatory signaling
Altered timing of reproductive hormone release
Reduced responsiveness of ovarian and uterine tissue
These changes are often adaptive from a survival perspective, but problematic for individuals attempting to conceive (2).
Sensitivity of the Reproductive System to Mild Thyroid Disruption
Importantly, fertility is sensitive to small shifts in thyroid function. Changes that may not meet diagnostic thresholds for hypothyroidism can still interfere with reproductive signaling (3).
This helps explain why some individuals experience fertility challenges despite having thyroid laboratory values within reference ranges. Reproductive tissues rely on local thyroid hormone availability and responsiveness, not just circulating hormone levels (1).
In this context, thyroid-related fertility issues are often functional and regulatory rather than structural or absolute.
How Hypothyroidism Can Disrupt Ovulation
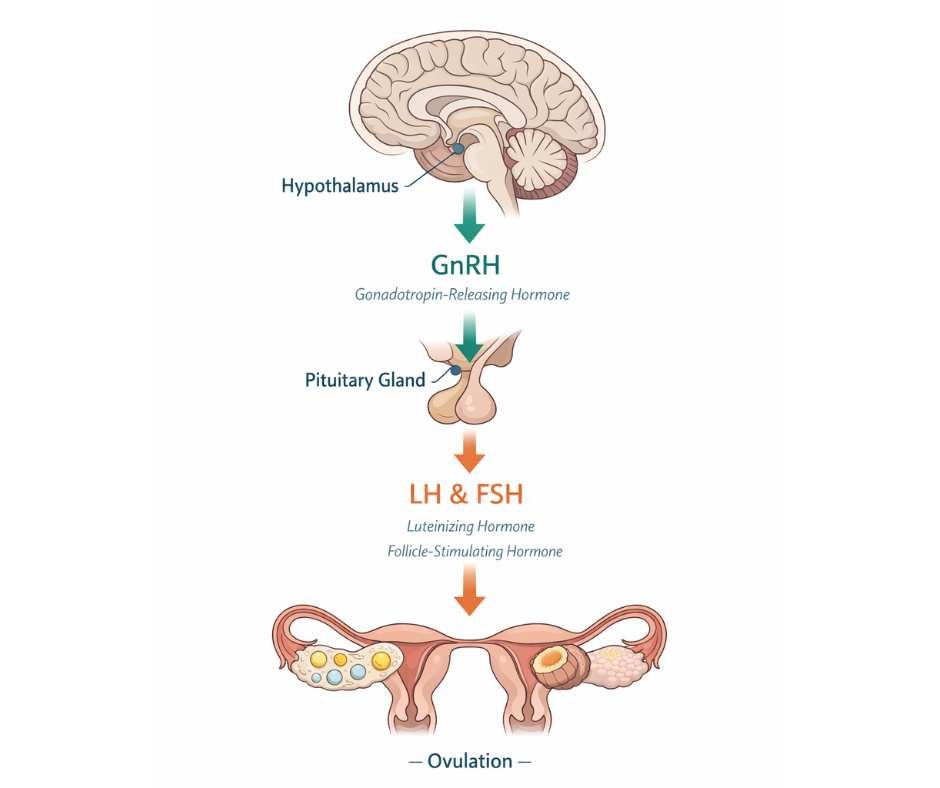
Ovulation depends on coordinated communication between the brain and the ovaries through a regulatory network known as the hypothalamic–pituitary–ovarian (HPO) axis. This system controls when an egg matures and is released each cycle.
At the top of this axis, the hypothalamus releases gonadotropin-releasing hormone (GnRH). GnRH acts as a timing signal, telling the pituitary gland when to release the hormones that stimulate the ovaries. The pituitary responds by releasing luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which drive follicle development and trigger ovulation.
Thyroid hormones influence this axis indirectly by regulating energy availability, metabolic signaling, and how responsive tissues are to hormonal cues. When thyroid hormone activity is reduced, the timing and strength of these reproductive signals can become less reliable—even when reproductive hormone levels appear within reference ranges (4).
Disruption of Brain–Ovary Signaling
In hypothyroidism, reduced thyroid hormone activity can interfere with HPO axis signaling by:
Altering the rhythmic release of GnRH from the hypothalamus
Reducing the pituitary’s responsiveness to GnRH
Disrupting the coordinated release of LH and FSH needed to support ovulation
When this signaling becomes inconsistent or blunted, ovulation may be delayed, irregular, or fail to occur altogether (5).
Functional Ovulatory Dysfunction
Importantly, hypothyroidism does not always result in complete anovulation. In many cases, ovulation still occurs—but in a functionally suboptimal way.
This may include:
Delayed ovulation, which shortens the fertile window
Weakened luteal phase support (the post-ovulation phase of the cycle)
Reduced progesterone production, which is necessary to support implantation
These patterns can lower the likelihood of conception and increase the risk of early pregnancy loss, even when cycles appear outwardly regular (6).
Why Ovulatory Issues Are Often Missed
Standard fertility evaluations often assess whether ovulation occurs at all, rather than whether it occurs consistently, predictably, and with adequate hormonal support.
Because hypothyroidism-related ovulatory disruption is frequently functional rather than absolute, it may not be detected unless thyroid signaling is evaluated within the broader context of reproductive regulation (7).
This helps explain why thyroid-related fertility challenges are often identified only after prolonged difficulty conceiving rather than during routine screening.
Thyroid Autoimmunity and Fertility Outcomes
Thyroid-related fertility challenges are not limited to hormone levels alone. In many cases, immune activity directed at the thyroid plays an independent role in reproductive outcomes.
Autoimmune thyroid conditions—most commonly Hashimoto’s thyroiditis—are characterized by the presence of thyroid-directed antibodies. These antibodies reflect ongoing immune activation rather than a static diagnosis, and their effects can extend beyond the thyroid gland itself (12).
How Thyroid Antibodies Influence Reproductive Physiology
Thyroid antibodies do not directly prevent conception, but they signal a broader immune environment that may be less supportive of reproduction.
Research suggests that autoimmune thyroid activity can influence fertility by:
Increasing systemic inflammatory signaling
Altering endometrial receptivity
Disrupting early pregnancy immune tolerance
These effects may occur even when thyroid hormone levels fall within reference ranges (13).
Importantly, antibody positivity reflects immune behavior, not irreversible tissue damage. The degree of immune activation can fluctuate over time based on regulatory inputs elsewhere in the body.
→ Immune Health & Autoimmune Support
Autoimmunity, Inflammation, and Early Pregnancy Loss
Successful implantation and early pregnancy require a carefully regulated immune response. The immune system must remain active enough to protect against infection while simultaneously allowing embryonic implantation to proceed.
In autoimmune thyroid conditions, immune signaling may shift toward heightened vigilance rather than tolerance. This shift has been associated with:
Increased risk of implantation failure
Higher rates of early pregnancy loss
Greater variability in fertility outcomes
These associations persist even when thyroid hormone replacement normalizes standard laboratory values (14).
Why Autoimmune Thyroid Patterns Are Often Overlooked in Fertility Care
Routine fertility evaluations may not assess thyroid antibodies unless overt thyroid disease is suspected. As a result, autoimmune thyroid activity may go unrecognized in individuals with otherwise unexplained fertility challenges.
Because autoimmune signaling can influence reproductive outcomes independently of hormone levels, its contribution may be missed unless immune regulation is considered as part of a broader fertility assessment (15).
This distinction helps explain why some individuals continue to experience fertility challenges despite appropriate thyroid medication and seemingly “normal” test results.
Why “Normal” Thyroid Labs May Not Protect Fertility
Standard thyroid testing is designed to assess hormone concentrations in the bloodstream. These values indicate whether thyroid hormone is present and circulating—but they do not necessarily reflect how effectively that hormone is being used by reproductive tissues (16).
Fertility depends on functional thyroid signaling, not simply hormone availability
Hormone Levels vs. Hormone Action
For thyroid hormones to support fertility, several steps must occur:
Thyroid hormones must be converted into their active form
They must enter ovarian and uterine cells
They must bind to responsive receptors
They must generate appropriate metabolic and signaling effects
Disruption at any of these steps can impair reproductive function without producing obvious abnormalities on routine blood tests (17).
This helps explain why fertility challenges may persist even when laboratory values fall within reference ranges.
Tissue-Level Sensitivity in Reproductive Organs
Ovarian and uterine tissues are particularly sensitive to thyroid hormone signaling. Even modest reductions in hormone activity at the cellular level can affect:
Follicle development
Endometrial maturation
Hormonal feedback involved in cycle timing
These tissue-level effects are not directly measured by standard thyroid panels, which focus on circulating hormone levels rather than local responsiveness (18).
The Limits of TSH-Centered Testing in Fertility Contexts
Thyroid-stimulating hormone (TSH) is commonly used as a screening marker, but it reflects pituitary signaling—not tissue-level thyroid activity.
In fertility contexts, reliance on TSH alone may overlook:
Impaired hormone conversion
Altered receptor sensitivity
Inflammatory or immune interference with signaling
As a result, individuals may be told their thyroid function is “normal” while reproductive tissues continue to experience functional thyroid insufficiency (19).
This disconnect underscores the importance of evaluating thyroid function in relation to fertility outcomes—not in isolation.
Why Thyroid Medication Alone May Not Restore Fertility
Thyroid hormone replacement can be an important part of care, but normalizing blood markers does not automatically restore fertility. This is because fertility depends on how thyroid hormones function at the tissue level—not simply on how much hormone is circulating in the bloodstream (20).
In many cases, medication improves laboratory values while the biological environment required for reproduction remains disrupted.
Medication Normalizes Levels, Not Regulation
Thyroid hormones support fertility only when they are:
Properly converted into active forms
Effectively taken up by ovarian and uterine tissue
Able to bind to responsive receptors
Operating within a stable immune and inflammatory environment
Medication addresses hormone availability, but it does not directly correct factors that interfere with hormone action, such as inflammation, immune activation, or metabolic stress (21).
As a result, reproductive signaling may remain impaired even when thyroid labs improve.
The Role of Inflammation and Immune Activity
Inflammation—particularly when driven by immune or autoimmune processes—can interfere with thyroid hormone signaling in multiple ways. It may:
Reduce conversion of thyroid hormone into its active form
Decrease tissue responsiveness to thyroid hormones
Shift metabolic signaling away from reproduction
These effects are protective from a survival standpoint but counterproductive for fertility. Increasing thyroid hormone dosage does not override these regulatory signals when inflammation remains active (22).
Why Fertility Outcomes May Lag Behind Lab Improvements
Reproductive tissues are highly sensitive to metabolic and immune conditions. Even subtle dysregulation can affect ovulation, implantation, or early pregnancy maintenance.
This helps explain why some individuals experience persistent fertility challenges despite being “well-controlled” on thyroid medication. The issue is not inadequate treatment—but incomplete regulation (23).
Restoring fertility often requires addressing the broader physiological context in which thyroid hormones operate, rather than relying on hormone replacement alone to correct downstream effects.
A Systems-Based Approach to Thyroid-Related Fertility Challenges
Fertility is not governed by a single hormone, organ, or laboratory value. It reflects the body’s overall regulatory state—how metabolic, immune, endocrine, and nervous system signals are coordinated in real time.
When thyroid dysfunction affects fertility, the issue is rarely limited to thyroid hormone production alone. More often, it involves how thyroid hormones are:
Converted and activated
Delivered to reproductive tissues
Interpreted within an immune and inflammatory context
Integrated with other reproductive hormones
This is why fertility challenges associated with thyroid dysfunction often persist when care focuses narrowly on hormone levels rather than on system-wide regulation.
A systems-based perspective recognizes that restoring fertility typically requires stabilizing the internal environment in which thyroid hormones operate—not simply correcting a single downstream marker (24).
This broader framework helps explain why addressing thyroid-related fertility challenges often involves looking beyond the thyroid itself and toward the interconnected systems that govern reproductive readiness.
How Thyroid Dysfunction Can Affect Fertility Outcomes Over Time
Thyroid-related fertility challenges rarely arise from a single abnormal lab value or isolated hormonal imbalance. Instead, they tend to reflect cumulative disruption across multiple regulatory systems that influence ovulation, cycle stability, implantation, and early pregnancy support.
When thyroid hormone signaling is impaired—whether due to reduced hormone activity, immune-mediated disruption, or altered tissue responsiveness—the reproductive system may receive inconsistent or suboptimal signals. Over time, this can affect fertility outcomes even in individuals who appear otherwise healthy and whose thyroid labs fall within reference ranges.
This helps explain why fertility challenges associated with thyroid dysfunction often persist unless evaluation extends beyond hormone levels alone. Metabolic regulation, immune balance, inflammatory signaling, and tissue-level hormone action all shape how thyroid hormones influence reproductive function.
Approaching thyroid-related fertility concerns through a systems-based lens allows these interconnected factors to be assessed together, rather than treating thyroid function as an isolated variable.
→ Thyroid Health & Hormone Balance
You may request a free 15-minute consultation with Dr. Martina Sturm to review your health concerns and outline appropriate next steps within a root-cause, systems-based framework.
Frequently Asked Questions About Thyroid Disorders and Fertility
Can hypothyroidism prevent pregnancy?
Hypothyroidism can make conception more difficult by disrupting ovulation timing, menstrual cycle regulation, and the hormonal signals required for implantation. Thyroid hormones interact with the brain–ovary axis and help regulate follicle development, luteal phase support, and endometrial preparation. Many people still become pregnant with hypothyroidism, but fertility outcomes are more consistent when thyroid signaling is stable at both the hormonal and tissue levels.
Can thyroid problems cause infertility even if my periods are regular?
Yes. Regular menstrual cycles do not guarantee optimal ovulation quality, adequate progesterone production, or healthy endometrial receptivity. Thyroid dysfunction can impair tissue-level hormone signaling and mitochondrial energy availability within the ovaries and uterus, affecting fertility physiology even when cycles appear predictable on the surface.
What thyroid level is best for getting pregnant?
There is no single thyroid value that guarantees fertility. While conventional care often focuses on TSH, fertility depends on how thyroid hormones are converted, transported, and received by reproductive tissues. A fertility-focused evaluation typically considers thyroid markers in context—alongside ovulatory patterns, symptoms, metabolic status, and reproductive history—rather than relying on one number alone.
Can Hashimoto’s thyroid antibodies affect fertility?
Thyroid antibodies are associated with altered immune signaling and increased inflammatory activity, both of which can influence implantation and early pregnancy support. Even when thyroid hormone levels are within reference ranges, immune activation reflected by antibodies may interfere with endometrial receptivity or early placental development. Their impact depends on the broader immune environment, not antibody presence alone.
Can thyroid issues cause miscarriage?
Thyroid dysfunction and thyroid autoimmunity have been associated with an increased risk of early pregnancy loss in some individuals. Unstable thyroid signaling can affect early placental development, while immune activation may disrupt implantation tolerance. Risk varies by individual, and outcomes often depend on how well thyroid and immune regulation are supported before and during early pregnancy.
Why am I still not getting pregnant if my thyroid labs are “normal”?
Standard thyroid labs measure hormone levels in the bloodstream, but fertility depends on hormone conversion, cellular responsiveness, and immune–inflammatory balance at the tissue level. If thyroid hormones are not being efficiently activated or received by reproductive tissues, fertility can be affected even when lab values fall within reference ranges. This disconnect explains why some individuals experience fertility challenges despite “normal” thyroid results.
Does thyroid medication improve fertility?
Thyroid medication can improve fertility in many cases by supporting hormone availability and stabilizing circulating levels. However, medication alone does not address all factors that influence tissue-level thyroid signaling, such as inflammation, immune activation, stress physiology, or metabolic strain. When these factors remain unaddressed, fertility challenges may persist despite appropriate dosing.
Should I test thyroid antibodies when trying to conceive?
In many cases, yes—particularly with unexplained infertility, a history of miscarriage, autoimmune conditions, or persistent symptoms despite normal thyroid labs. Antibody testing can help identify immune-driven thyroid patterns that may influence implantation or early pregnancy, allowing evaluation to extend beyond hormone levels alone.
Still Have Questions?
If the topics above reflect ongoing symptoms or unanswered concerns, a brief conversation can help clarify whether a root-cause approach is appropriate.
Resources
Endocrine Reviews – Thyroid hormone action at the cellular level
Endocrine Reviews – Thyroid function and female reproductive physiology
The Journal of Clinical Endocrinology & Metabolism – Subclinical hypothyroidism and ovulatory dysfunction
Human Reproduction – Menstrual irregularities associated with thyroid disorders
Fertility and Sterility – Thyroid hormones and endometrial receptivity
Human Reproduction Update – Thyroid autoimmunity and fertility outcomes
The Journal of Clinical Endocrinology & Metabolism – Limitations of standard thyroid testing in reproductive health
Fertility and Sterility – Thyroid hormone replacement and reproductive outcomes
Endocrine Reviews – Hypothalamic–pituitary–ovarian axis regulation
Gynecological Endocrinology – Luteal phase deficiency and thyroid dysfunction
Nature Reviews Endocrinology – Tissue-level thyroid hormone sensitivity
Endocrine Reviews – Inflammatory signaling and reproductive hormone function
The Journal of Clinical Endocrinology & Metabolism – Thyroid antibodies and early pregnancy loss
Trends in Endocrinology & Metabolism – Immune tolerance and implantation
Human Reproduction Update – Autoimmune thyroid disease and reproductive outcomes
Nature Reviews Endocrinology – Circulating thyroid hormones versus cellular utilization
Endocrine Reviews – Thyroid hormone conversion and fertility
Molecular Endocrinology – Thyroid receptor sensitivity in reproductive tissues
The Journal of Clinical Endocrinology & Metabolism – Clinical limitations of TSH-centered thyroid assessment
Trends in Endocrinology & Metabolism – Inflammation and thyroid hormone signaling
Endocrine Reviews – Immune-mediated thyroid dysfunction
Endocrine Reviews – Metabolic stress and reproductive suppression
Fertility and Sterility – Thyroid regulation and early pregnancy maintenance